Formulation Variety Can Be Designed Based on AGO™ Nanoparticle for Various Administration Routes
AGO™ nanoparticle for IV injection

AGO™-based injectable gel for SC injection

AGO™-based spray-dried powder (left: raw materials and right: curcumin encapsulated) for inhalation or oral (1-5 um size)
OPBI 1001:
Biologic Single Drug Formulation


Animal Study Result - TRASTUZUMAB (TRA) SC Injectable
7-week-old female Balb/c Nu mice with SKBR-3 xenograft
right flank SC injection ; 200 uL per dose; n=4

- The weight of the mice, subjecting under different T-mAb dosing controls, shows a stable increased profile, indicating the physiological condition of the mice kept good and healthy condition during the test.
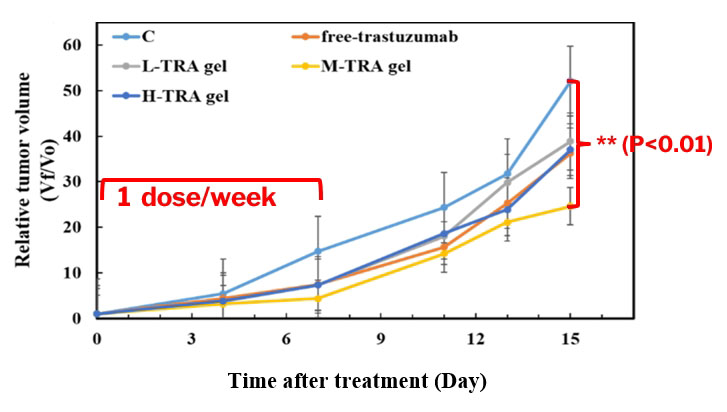
- The tumor volume change of the mice, subjecting under different T-mAb dosing controls with 1 dose/week, shows a significant growth inhibition behavior for the M-TRA gel protocol, indicating the best potency of this dosing control to inhibit SKBR-3 breast tumor development.
Animal Study Result – Anti-Cancer Pathological Analysis (Lung)
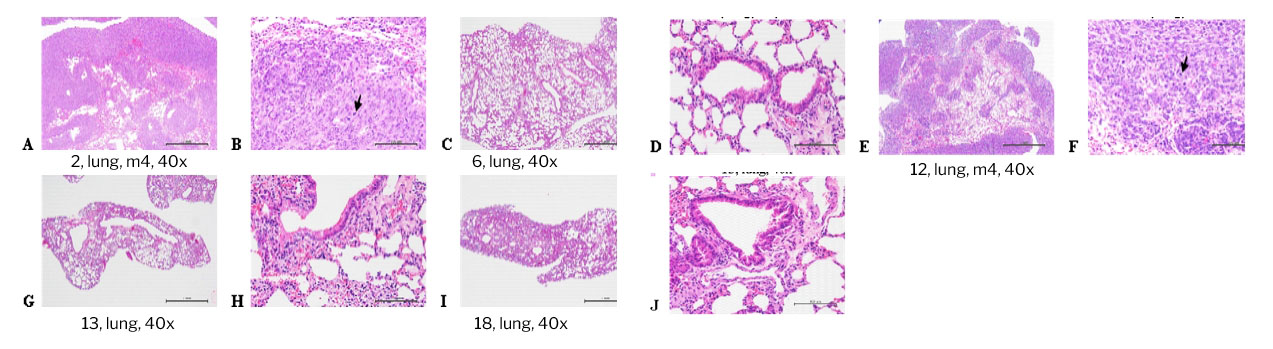
Histopathological findings of tumor masses of Balb/c nude mice implanted with xenograft SKBR-3 tumor cells in the right flank and subcutaneous injection of TRA gel, and mice were sacrificed on day 14. Multifocal, tumor cell metastasis with high levels of mitosis in the lungs (arrow) was graded moderate/severe (4) in the Control (A and B. animal code: 1), absent (0) in the free-trastuzumab (C and D. animal code: 6), moderate/severe (4) in the L-TRA gel (E and F. animal code: 12), absent (0) in the M-TRA gel (G and H. animal code: 13) and H-TRA gel (I and J. animal code: 18) groups. H&E stain. 40x and 400x.
Summary:
A tumor metastasis was found in the lung for Control group and L-TRA , but absent for free-TRA, M-TRA, and H-TRA groups, indicating the tumor inhibition efficacy can be achieved for our dosing protocols.
OPBI 1003:
Biologic & Chemo Synergistic Dual Drug Formulation


Biosimilar Drug + Chemo Drug Release Profiles
In-vitro release profile of paclitaxel from the dual-drug (chemo-drug + biosimilar drug) injectable gel showed a sustainable manner over a time period of 7 days from both AGO™ nanocarrier and associated AGO™ injectable gel. And PTX release rate showed a much slower profile once co-encapsulated with T-mAb within the AGO™ Gel.

Free PTX
PTX-Carrying AGO™ nanoparticle
PTX + T-mAb AGO™ injectable gel
PTX-TRA Dual-Drug Subcutaneous (SC) Dosing Protocol
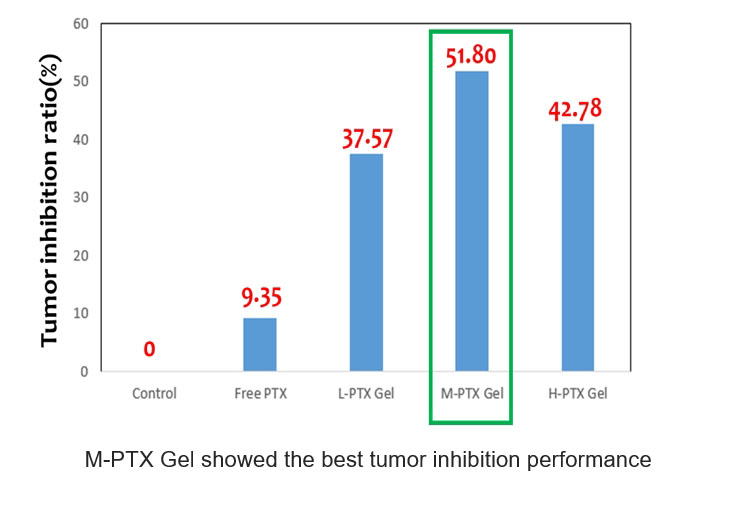
- Dual-drug SC dosing protocol showed that all the PTX-TRA-carrying gels demonstrated pronounced tumor inhibition behavior and the best anti-tumor efficacy was observed for M-TRA (with PTX:TRA=40:4 ratio)
- The result indicates that the dual-drug system (chemo drug + biosimilar drug) injectable designed was considerably improved anti-tumor performance compared to free dual-drug delivery (9.35%)

OPBI 1005:
T-mAb Targeting, PACLITAXEL-CURCUMIN Dual Drug


Dual-Drug (Chemo-Drugs) with T-mAb Targeting Co-Delivery
- In-vivo study showed that targeting dual-drug IV injection protocol exhibits the best tumor inhibition performance (with Cur:PTX=25:5 ratio)
- The result indicates that dual-drug system (chemo drug + chemo drug) designed was considerably improved anti-tumor performance (68.9%) compared to free dual-drug (60.2%) or single drug (48%) protocol to against HER2+ breast tumor

Dual-Drug (Chemo-Drugs) with Targeting Delivery (TRA)
- Tumor growth was highly inhibited with the use of dual-drug targeting AGO delivery (using T-mAb as targeting moiety) against HER2-positive highly malignant breast cancer.

